Abstract
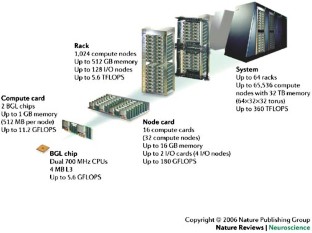
IBM's Blue Gene supercomputer allows a quantum leap in the level of detail at which the brain can be modelled. I argue that the time is right to begin assimilating the wealth of data that has been accumulated over the past century and start building biologically accurate models of the brain from first principles to aid our understanding of brain function and dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
The Blue Brain Project [online], <http://bluebrainproject.epfl.ch> (2005).
Blue Gene [online], <http://www.research.ibm.com/bluegene> (2005).
Deep Blue [online], <http://www.research.ibm.com/deepblue> (2005).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952).
Rall, W. Branching dendritic trees and motoneuron membrane resistivity. Exp. Neurol. 1, 491–527 (1959).
Segev, I. & Rall, W. Excitable dendrites and spines: earlier theoretical insights elucidate recent direct observations. Trends Neurosci. 21, 453–460 (1998).
Johnston, D. et al. Active dendrites, potassium channels and synaptic plasticity. Phil. Trans. R. Soc. Lond. B 358, 667–674 (2003).
Magee, J. C. Dendritic integration of excitatory synaptic input. Nature Rev. Neurosci. 1, 181–190 (2000).
London, M. & Hausser, M. Dendritic computation. Annu. Rev. Neurosci. 28, 503–532 (2005).
Migliore, M. & Shepherd, G. M. Emerging rules for the distributions of active dendritic conductances. Nature Rev. Neurosci. 3, 362–370 (2002).
Rall, W. & Shepherd, G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J. Neurophysiol. 31, 884–915 (1968).
Pellionisz, A., Llinas, R. & Perkel, D. H. A computer model of the cerebellar cortex of the frog. Neuroscience 2, 19–35 (1977).
Shepherd, G. M. & Brayton, R. K. Computer simulation of a dendrodendritic synaptic circuit for self- and lateral-inhibition in the olfactory bulb. Brain Res. 175, 377–382 (1979).
Traub, R. D. & Wong, R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science 216, 745–747 (1982).
Traub, R. D., Miles, R. & Buzsaki, G. Computer simulation of carbachol-driven rhythmic population oscillations in the CA3 region of the in vitro rat hippocampus. J. Physiol. (Lond.) 451, 653–672 (1992).
Douglas, R. J. & Martin, K. A. C. A functional microcircuit for cat visual cortex. J. Physiol. (Lond.) 440, 735–769 (1991).
Wang, X. J. & Rinzel, J. Spindle rhythmicity in the reticularis thalami nucleus: synchronization among mutually inhibitory neurons. Neuroscience 53, 899–904 (1993).
De Schutter, E. & Bower, J. M. An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J. Neurophysiol. 71, 375–400 (1994).
Bush, P. & Sejnowski, T. J. Inhibition synchronizes sparsely connected cortical neurons within and between columns in realistic network models. J. Comput. Neurosci. 3, 91–110 (1996).
Contreras, D., Destexhe, A., Sejnowski, T. J. & Steriade, M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274, 771–774 (1996).
Destexhe, A., Bal, T., McCormick, D. A. & Sejnowski, T. J. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J. Neurophysiol. 76, 2049–2070 (1996).
Golomb, D. & Amitai, Y. Propagating neuronal discharges in neocortical slices: computational and experimental study. J. Neurophysiol. 78, 1199–1211 (1997).
Lytton, W. W., Contreras, D., Destexhe, A. & Steriade, M. Dynamic interactions determine partial thalamic quiescence in a computer network model of spike-and-wave seizures. J. Neurophysiol. 77, 1679–1696 (1997).
Destexhe, A., Contreras, D. & Steriade, M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience 92, 427–443 (1999).
Egger, V., Feldmeyer, D. & Sakmann, B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nature Neurosci. 2, 1098–1105 (1999).
Bal, T., Debay, D. & Destexhe, A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. J. Neurosci. 20, 7478–7488 (2000).
Howell, F. W., Dyrhfjeld-Johnsen, J., Maex, R., Goddard, N. & De Schutter, E. A large scale model of the cerebellar cortex using PGENESIS. Neurocomputing 32, 1036–1041 (2000).
Kozlov, A., Kotaleski, J. H., Aurell, E., Grillner, S. & Lansner, A. Modeling of substance P and 5-HT induced synaptic plasticity in the lamprey spinal CPG: consequences for network pattern generation. J. Comput. Neurosci. 11, 183–200 (2001).
Bazhenov, M., Timofeev, I., Steriade, M. & Sejnowski, T. J. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J. Neurosci. 22, 8691–8704 (2002).
Pinto, D. J., Jones, S. R., Kaper, T. J. & Kopell, N. Analysis of state-dependent transitions in frequency and long-distance coordination in a model oscillatory cortical circuit. J. Comput. Neurosci. 15, 283–298 (2003).
Bazhenov, M., Timofeev, I., Steriade, M. & Sejnowski, T. J. Potassium model for slow (2–3 Hz) in vivo neocortical paroxysmal oscillations. J. Neurophysiol. 92, 1116–1132 (2004).
Traub, R. D. et al. Single-column thalamocortical network model exhibiting gamma oscillations, sleep spindles, and epileptogenic bursts. J. Neurophysiol. 93, 2194–2232 (2005).
Thomson, A. M., Girdlestone, D. & West, D. C. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer III pyramidal neurons in rat neocortical slices. J. Neurophysiol. 60, 1896–1907 (1988).
Dodt, H. U. & Zieglgansberger, W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 537, 333–336 (1990).
Stuart, G. J., Dodt, H. U. & Sakmann, B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 423, 511–518 (1993).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nature Rev. Neurosci. 5, 793–807 (2004).
Martin, K. A. Microcircuits in visual cortex. Curr. Opin. Neurobiol. 12, 418–425 (2002).
Silberberg, G., Gupta, A. & Markram, H. Stereotypy in neocortical microcircuits. Trends Neurosci. 25, 227–230 (2002).
Toledo-Rodriguez, M. et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb. Cortex 14, 1310–1327 (2004).
NEURON [online], <http://www.neuron.yale.edu/neuron> (2005).
Tsodyks, M., Pawleslik, K. & Markram, H. Neural networks with dynamic synapses. Neural Comput. 10, 821–835 (1998).
NeoCortical Simulator [online], <http://brain.cse.unr.edu/ncsDocs> (2005).
SGI [online], <http://www.SGI.com> (2005).
The Human Brain Project [online], <http://www.nimh.nih.gov/neuroinformatics> (2005).
Markram, H. Dendritic object theory: a theory of the neural code where 3D electrical objects are formed across dendrites by neural microcircuits. Swiss Soc. Neurosci. Abstr. 196 (2005).
Acknowledgements
I am grateful for the efforts of all my students, especially Y. Wang, A. Gupta, M. Toledo and G. Silberberg, in carrying out such challenging experiments and producing such incredible data. I thank P. Aebischer, G. Margaritondo, F. Avellan, G. Parisod and the entire EPFL (Ecole Polytechnique Fédérale de Lausanne) administration for their support of this project and for acquiring Blue Gene. I thank IBM (International Business Machines) for making this prototype supercomputer available and for their major support of neuroscience. I also thank SGI (Silicon Graphics, Inc.) for their major initiative to help with the visualization of the Blue Brain. I thank P. Goodman for his long-standing support of our reconstruction efforts and for introducing me to the Blue Gene initiative in 2000. Thanks also to the US Office of Naval Research for their support. I thank I. Segev, who is and will be essential to the success of the project, and G. Shepherd for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Markram, H. The Blue Brain Project. Nat Rev Neurosci 7, 153–160 (2006). https://doi.org/10.1038/nrn1848
Issue Date:
DOI: https://doi.org/10.1038/nrn1848
This article is cited by
-
Recent advances in imaging devices: image sensors and neuromorphic vision sensors
Rare Metals (2024)
-
Multiple neuron clusters on Micro-Electrode Arrays as an in vitro model of brain network
Scientific Reports (2023)
-
Uncertainty quantification and sensitivity analysis of a hippocampal CA3 pyramidal neuron model under electromagnetic induction
Nonlinear Dynamics (2023)
-
Petabyte-Scale Multi-Morphometry of Single Neurons for Whole Brains
Neuroinformatics (2022)
-
The Neuron Phenotype Ontology: A FAIR Approach to Proposing and Classifying Neuronal Types
Neuroinformatics (2022)